The greatest challenge of the proposed "Exponential Remediation of Civilization's Footprint" is the necessary sequestration of chlorine evolved during the production of concrete, from oceanic salt ions (Ca++, Na+, Cl- and CO3--), for 70,000 atolls. The cited Calera process concrete produces 71% of its weight in chlorine (see "Comparison With Land Based Geologic Sequestration of CO2" below) while sequestering CO2 in the atoll concrete.
Geologic Sequestration
Later, we'll compare the magnitude of chlorine to the magnitude of land-based geologic sequestration of CO2, which can support many times the CO2 projected to be sequestered in the atolls. But that requires transportation of evolved chlorine to those sites. So first, we'll look at the in situ potential for geologic sequestration.
In situ resource utilization is highly desirable in an exponentially growing system. The civil engineering sense of "in situ" is applicable: "construction which is carried out at the building site using raw materials... which are present at or near a project site". In situ resources obviate their transportation cost which, in exponential growth, can represent a severe constraint. In the present case, resources include not only those that go into the atoll concrete, but also the resources to dispose of waste: geologic formations under the tropical doldrums suitable for chlorine sequestration.
In situ resource utilization is highly desirable in an exponentially growing system. The civil engineering sense of "in situ" is applicable: "construction which is carried out at the building site using raw materials... which are present at or near a project site". In situ resources obviate their transportation cost which, in exponential growth, can represent a severe constraint. In the present case, resources include not only those that go into the atoll concrete, but also the resources to dispose of waste: geologic formations under the tropical doldrums suitable for chlorine sequestration.
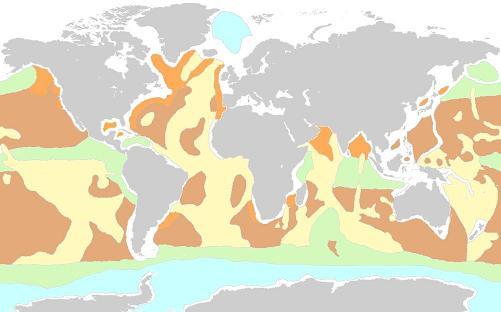
Look at this map of deep sea marine sediments.
very fine grained, suitable for EveCrete binding) and calcium carbonate sediments, respectively, available in situ.
Another map disagrees somewhat but substantially supports the general point:
Sand is used in the concrete. Calcium carbonate sediments lying about 1000ft beneath the ocean floor, offer virtually ideal chlorine sequestration via the reaction:
CaCO3 + 2HCl => CaCl2 + H2CO3 + heat
Chlorine sequestration is thereby turned into H2CO3 (carbonic acid) sequestration, 1000ft beneath the already several-kilometer deep ocean floor. The H2CO3 will act as a connate fluid that is gradually expressed from the sediment, upward toward the ocean floor 1000ft above, over geologic time during lithification: the process turning sediments to sedimentary rock. Unlike water, the usual connate fluid, this particular connate fluid chemically interacts with the CaCO3 sediments via carbonate buffering. This provides additional environmental protections in the form of pH stabilization and slowing of the already geologic time rate of reentry to the biosphere.
Is There Enough Sediment To Contain All That H2CO3?
The volume of in situ calcareous sediments is on the order of 10,000 times greater than the total volume of H2CO3 evolved (which is comparable to the volume of Cl2 evolved from the 70,000 atolls). If only 1% of that volume is utilized for geologic sequestration, the volumetric concentration would still be only 1% of that reduced volume.
How Costly Is the Geologic Sequestration?
Existing deep sea drilling technology suffices as an economic existence proof. A deep sea drilling platform costs about 10% of the net present value of an atoll. Even if each atoll requires its own drilling platform, this is not blocking. If chlorine is delivered to the sediment with ocean floor of about 12,000ft, liquid chlorine density of 1.5625gm/ml will provide about 8,000psi over-pressure at the ocean floor due to its higher density than water.
12000ft;1.5625g/ml?psi
(12000 * foot) * ([1.5625 * gramf] / [milli*liter]) ? psi
= 8128.6473 psi
This is well within the engineering limits of deep sea drilling. Going 1000ft deep into CaCO3 sediments will subtract about 1,000psi from that injection pressure.
1000ft;2.7g/ml?psi
(1000 * foot) * ([2.7 * gramf] / [milli*liter]) ? psi
= 1170.5252 psi
The compressive strength of concrete is only about 5,000psi so even if the CaCO3 sediment is in the form of concrete, the injection pressure at that depth will combine with the corrosive nature of Cl2 to fracture the sediments, permitting the ingress of liquid chlorine.
The connate fluid already in the sediments will be dominated by H2O, thereby producing HCl via the reaction:
2Cl2 + 3H2O => 5HCl + HClO3 + heat
It is this HCl that will participate in the reaction already described that produces H2CO3.
12000ft;1.5625g/ml?psi
(12000 * foot) * ([1.5625 * gramf] / [milli*liter]) ? psi
= 8128.6473 psi
This is well within the engineering limits of deep sea drilling. Going 1000ft deep into CaCO3 sediments will subtract about 1,000psi from that injection pressure.
1000ft;2.7g/ml?psi
(1000 * foot) * ([2.7 * gramf] / [milli*liter]) ? psi
= 1170.5252 psi
The compressive strength of concrete is only about 5,000psi so even if the CaCO3 sediment is in the form of concrete, the injection pressure at that depth will combine with the corrosive nature of Cl2 to fracture the sediments, permitting the ingress of liquid chlorine.
The connate fluid already in the sediments will be dominated by H2O, thereby producing HCl via the reaction:
2Cl2 + 3H2O => 5HCl + HClO3 + heat
It is this HCl that will participate in the reaction already described that produces H2CO3.
Comparison With Land Based Geologic Sequestration of CO2
As it turns out, the liquid volume of CO2 sequestered in the concrete of the artificial atolls is about the same as the liquid volume of Cl2 produced.
100000people/atoll;7e9people;20km/atoll;(20/100)*2000tonne/m?tonne
= 5.6E11 tonne
Total CaCO3 mass of atolls.
(12/100)*5.6E11 tonne?tonne (12 / 100) * (5.6E11 * ton_metric) ? ton_metric
= 6.72E10 tonne
Total C mass of atolls.
(40/100)*5.6E11 tonne?tonne (40 / 100) * (5.6E11 * ton_metric) ? ton_metric
= 2.24E11 tonne
Total Ca mass of atolls.
= 2.24E11 tonne
Total Ca mass of atolls.
321003271mi^3;0.04%*1020kg/m^3?tonne
= 5.45904E14 tonne
= 5.45904E14 tonne
Total Ca mass in the entire ocean.
(2*35.45/100)*5.6E11 tonne?tonne
= 3.9704E11 tonne
= 3.9704E11 tonne
Total Cl2 mass evolved during atoll construction from Calera process.
(44/100)*5.6E11 tonne?tonne
= 2.464E11 tonne
Total CO2 mass sequestered during atoll construction from Calera process.
= 2.464E11 tonne
Total CO2 mass sequestered during atoll construction from Calera process.
1.5625g/cm^3;3.9704E11?m^3
= 2.541056E11 m^3
Total (liquid) Cl volume as geologically sequestered (prior to mineralization)
= 2.541056E11 m^3
Total (liquid) Cl volume as geologically sequestered (prior to mineralization)
1101 kg/m^3;2.464E11 tonne?m^3
= 2.2E11 m^3
Total (liquid) CO2 volume as it would have been geologically sequestered (prior to mineralization)